| Fig.1 |
Macrocrystalline quartz basically forms in three geological environments:
In these environments individual crystals grow by adding molecules at their surface, layer by layer[1].
Quartz crystals typically grow in hot watery solutions, in so-called hydrothermal environments, at temperatures between 100°C and 450°C and often at very high pressures. There are no free SiO2 molecules in such a watery solution, instead, quartz crystals grow by addition of dissolved orthosilicic acid, H4SiO4 at their surface, giving off water, layer by layer. The general reaction formula already given in the chapter Chemical Properties is:
The dissolution of quartz in water is just the opposite chemical process, leading to the formation of orthosilicic acid:
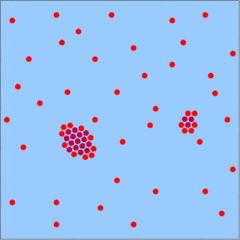
The following figures schematically describe the growth process in a watery solution.

|
Should the temperature or the pressure fall, the solubility of silica decreases and the solution will get saturated with respect to quartz. If there are quartz grains in adjacent rocks, the H4SiO4 molecules will be added to them and the crystals will grow into the solution. If the adjacent rocks are void of quartz grains, the H4SiO4 molecules will aggregate to form small floating crystals that will bind more and more H4SiO4 molecules. This spontaneous formation of crystals is only possible in a well oversaturated solution. SiO4 tetrahedra that have been fully incorporated into the quartz crystal are symbolized by dark red dots.

|
The formation of new crystals is not inhibited as such, but in conditions of slow growth the larger crystals are favored. The reason is that growth is not simply the result of constant addition of molecules to a crystal. It is the net result of both addition and removal of molecules. Molecules tend to get removed from the edges of the crystals, and the relation of the number of edges to the overall volume of the crystal is not in favor of small crystals. Small crystals will not grow very fast and might even get dissolved again.
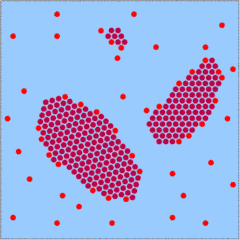
|

|
Because crystals enclose drops of the surrounding watery solution during growth, it is possible to reconstruct the changing environmental conditions. Such studies have shown that in many crystals growth apparently started at about 250-450°C and came to a halt at temperatures still well above 100°C.
Falling temperatures and/or pressures are essential conditions for quartz crystal growth. What causes the decrease in pressure and temperature?
This depends on the geological environment. If hot brines from deeper rocks quickly enter rocks at the surface via cracks, they will cool very quickly. The result is a rapid precipitation of silica in the cracks and the formation of massive white vein or gangue quartz[1].
If, however, the watery solutions are enclosed in a cavity inside the rocks, the solution will cool just as fast or slow as the host rock. For example, hot magma that intrudes in a rock will heat up its vicinity. Depending on the size of the magmatic body, the cooling of the body and the adjacent rocks can take hundreds or hundred thousands of years.
The most interesting temperature changes for quartz collectors are those associated with an uplift of large bodies of rock due to tectonic activity. When crustal plates collide, large parts of the earth's crust are bent and lifted up several kilometers from their initial position, in particular in the case of continent-continent collisions. Rocks appear at the surface that once have been up to more than 50 kilometers below the surface. The temperature at these depths is about 800-1200°C, and at a depth of 20 kilometers it is still around 200-400°C. If a water-filled cavity is lifted up together with the surrounding rock, it can take millions of years until it reaches the surface, and of course it will take millions of years to cool down. Under such conditions - which are met in alpine-type clefts, for example - quartz crystals of exceptional size and clarity can form.
Quartz crystals often do not grow continuously, but in short phases. This pulsating growth is sometimes explained with tectonic activity, causing a sudden uplift of the host rock and a relatively fast drop in pressure.
O- O- O- OH O- O- O-
| | | | | | |
...-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O...
| | | | | | |
O- O- OH O- O- O- O-
low water content
O- OH O- OH O- OH OH
| | | | | | |
...-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O...
| | | | | | |
OH OH OH O- O- OH O-
high water content
These flexible molecules swim in the molten rock like spaghetti in a thick sauce of other ions. They are the cause for the high viscosity of lava, and the more silica a rock contains, the more viscous is its melt. Like polysilicic acids in a watery solution, they carry negative charges and hydrogen atoms in hydroxyl groups (-OH), but the relation of [O-] to [OH] depends on the amount of water dissolved in the magma.
When molten magma cools rapidly, typically at a volcanic eruption, the time is too short for the bonds in the SiO4-chains to break up and new bonds to form in ordered structures of crystallized silicates. The "pasta-like" random structure of the melt is then preserved in a volcanic glass, like the glassy obsidian, or the sponge-like pumice. High viscosity is a hindrance to crystal growth, so silica rich lavas (for example, rhyolites and andesites) have a greater tendency to form volcanic glasses.
When the magma intrudes other rocks but doesn't erupt and stays inside the earth's crust, it cools very slowly. Crystals of different minerals will appear in the melt in an order that is primarily determined by the chemical composition of the magma. In a granite, for example, mica crystals are the first to form, followed by feldspars, and finally quartz. Because they are usually the last to form and simply fill out the remaining voids in the rock, you will rarely see ideally-shaped quartz crystals in a granite, while well-formed crystals of feldspar can be found frequently.
Quartz crystals in igneous rocks occasionally show an onion-like internal structure, indicating a pulsating addition of outer layers to the crystals. Often the chemical properties of included minerals change gradually from the core to the surface of a crystal, reflecting a gradual change in the chemistry of residual liquid parts in the solidifying magma.
Suppose we put a quartz crystal into a saturated watery solution of orthosilicic acid. Will the crystal grow as the water evaporates, like salt crystals do in salt brine? It primarily depends on the temperature: at room temperature, silicic acids have a strong tendency to polymerize, although the solubility of orthosilicic acid is very low. And in fact sometimes quartz crystals are found that have an opal or chalcedony cap. The speed of polymerization has surpassed the speed of growth of the crystal at that temperature.
The following table lists factors that promote or inhibit the formation of either macrocrystalline or cryptocrystalline quartz.
| Promoting Factors | Inhibiting Factors | |
|---|---|---|
| Macrocrystalline |
|
|
| Cryptocrystalline |
|
|
There is no clear line that separates the conditions of crypto- or macrocrystalline quartz formation, and there is not a single determining factor.
Other factors, like the pressure, may also play a role. One factor is clearly inhibiting the formation of cryptocrystalline quartz: the absence of liquid water - cryptocrystalline quartz is not a primary constituent of magmatic rocks like granite or basalt. The chalcedony that is commonly found in basalt is a secondary product of alterations of the host rock under the influence of water.
|
Source: http://www.quartzpage.de/print/gen_form.html |
Home: www.quartzpage.de |