last modified: Friday, 03-Oct-2014 01:10:52 CEST
Document status: unfinished, but usable
Introduction
 1600x1888 685kb
1600x1888 685kb
|
Under conditions at or near the surface, quartz is more stable than most other minerals and assumes a mostly passive role in the geological environment, and this is the reason why the billion years old quartz crystals from Brazil look just as fresh and new as Alpine rock crystals that formed "just" 10 million years ago.
But while it is chemically an almost inert and passive substance at the surface, quartz is a very active agent under conditions deep within the Earth's crust. Here, at higher temperatures and pressures, it participates in many complex chemical reactions during rock and mineral formation.
The image to the right has been made at Zion National Park, Utah, U.S.A., and shows sandstone made of "fossilized" Triassic sand dunes that has weathered into bizarre shapes. These rocks consist almost entirely of quartz.
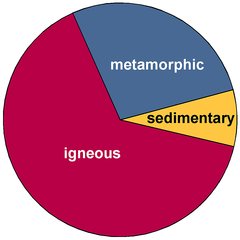
Distribution of Quartz in Different Types of Rocks
Quartz is very unevenly distributed: Some rocks are entirely made of quartz, others are completely void of it. The rocks of the Earth's crust are subdivided into 3 classes (volume data from Okrusch and Matthes, 2005, after Ronov and Yaroshevsky, 1969):
|
- Igneous Rocks - rocks that originate from magma and that accordingly are also called magmatic rocks. Either they cool and solidify slowly inside the Earth's crust, then they are called intrusive igneous rocks or plutonic rocks. Or they cool quickly at or near to the surface, then they are called extrusive igneous rocks or, more commonly, volcanic rocks. It is estimated that igneous rocks make up 64.7% of the volume of the Earth's crust.
- Sedimentary Rocks - rocks that are formed from deposited material. Most of the Earth's crust is covered by sedimentary rocks and unsolidified sediments like sand, but the contribution to its overall volume is low: just 7.9% of the Earth's crust is made of sedimentary rocks.
- Metamorphic Rocks - rocks which are derived from both sedimentary and igneous rocks by exposure to temperatures and/or pressures that are different from the conditions of their initial formation. This is a very diverse class of rocks with a wide range of mineral compositions. Metamorphic rocks are estimated to contribute 27.4% to the volume of the Earth's crust.
The largest amount of quartz is contained in igneous rocks, in particular in so-called granitoids, granites and related rocks. When one talks about the Earth's crust as a whole, volcanic rocks are more common at the surface than granitoids, because that encompasses the oceanic crust that is largely made of basalt and gabbro. These rocks have a very low quartz content or are void of it. On the other hand, the continental crust contains large amounts of granitic rocks that are rich in quartz, so almost all quartz is found in the continental crust that covers just about 40% of the Earth's surface[1].
Very high concentrations of quartz can be found in certain sedimentary rocks like sandstone, as well as in alluvial[2] and marine sands and sand dunes. On the other hand, limestones are also very common and typically have a very low quartz content. The abundance of sandstones and sands at the Earth's surface is a bit misleading, as sedimentary rocks cover most of the Earth, but only make up a small part of the Earth's crust volume, so the total amount of quartz in them is rather low.
Metamorphic rocks also show large variations in quartz content. The quartz content often reflects the mineral composition of the precursor rock. Very high concentrations of quartz are found quartzite and certain schists, while rocks derived from limestones and certain igneous rocks may be void of it. The total amount of quartz present in metamorphic rocks is lower than in igneous but higher than in sedimentary rocks. Metamorphosis is the only major process in which quartz is either produced or consumed and disappears from the environment during the formation of new minerals.
Finally, quartz and all the other silica polymorphs are absent from the Earth's upper mantle rocks, and the presence of silicon dioxide polymorphs stishovite and seifertite that are stable under these conditions in the lower mantle is uncertain.
Quartz Content and Silica Content of Rocks
Due to the importance of quartz in geochemical processes, rocks are often classified both by their quartz content and by their silica content. Quartz is one form of silica, SiO2, but the terms "quartz content" and "silica content" have a very different meaning.
- quartz content means the relative content of the mineral quartz in a rock.
- silica content means the relative content of SiO2 in the total chemical composition of a rock or a mineral.
For example, a rock that is entirely made of albite, NaAlSi3O8, a feldspar, has 0% quartz content, but contains 77.3% silica (weight percent).
It was once customary to express the chemical composition of a mineral as an oxide formula (mostly because the structural relationships between the elements involved were not precisely known), and it is still useful if one reflects the silica content. So in the case of albite, instead of
NaAlSi3O8
one could as well write:
Na2O ⋅ Al2O3 ⋅ 6 SiO2
which reads as 1 part sodium oxide, 1 part aluminum oxide, and 6 parts silicon oxide, silica.
Or, maintaining the number of atoms in the original formula:
0.5 Na2O ⋅ 0.5 Al2O3 ⋅ 3 SiO2
And in fact, one could take 1 part of Na2O, 1 part of Al2O3 and 6 parts of SiO2, put them in a pot, turn on the heat, and after some time one would get albite.
- the compound SiO2 dissolved in fluids or magma that would either turn into quartz (or another silica polymorph) or enter chemical reactions with other minerals upon changes in temperature or pressure;
- any of the silica polymorphs (like quartz, cristobalite, stishovite, etc.) present as a mineral.
Silica and quartz content in an imaginary rock
To get a feeling for the numbers used in rock classification, I have calculated the silica content of an imaginary rock made of 20% quartz, 60% orthoclase feldspar and 20% muscovite mica, and presented the results in Table 2.1 below. The composition of this rock is a bit simple, but it would qualify as a so called "alkali-feldspar granite".
| Regular Formula |
Oxide Formula |
Molar Weight |
Weight Percent |
Contribution to Total Composition | |
|
Quartz 20% | SiO2 | 1.0 SiO2 | 60.0843 | 100% | × 20% = 20% SiO2 |
|
Orthoclase 60% | KAlSi3O8 |
0.5 K2O 0.5 Al2O3 3.0 SiO2 |
94.1906 101.96128 60.0843 |
16.9% 18.3% 64.8% |
× 60% = 10.1% K2O × 60% = 11.0% Al2O3 × 60% = 38.9% SiO2 |
|
Muscovite 20% |
KAl2[(OH)2AlSi3O10] |
0.5 K2O 1.5 Al2O3 3.0 SiO2 1.0 H2O |
94.1906 101.96128 60.0843 18.0152 |
11.8% 38.4% 45.3% 4.5% |
× 20% = 2.4% K2O × 20% = 7.6% Al2O3 × 20% = 9.1% SiO2 × 20% = 0.9% H2O |
|
Total 100% |
= 12.5% K2O = 18.6% Al2O3 = 68.0% SiO2 = 0.9% H2O |
So if we sum up the oxide components of this imaginary rock, we get 68.0% silica, SiO2, although the rock contains only 20% quartz, or 20% free silica.
Table 2.2: Mol percent versus weight percent values for the imaginary rock.
Fig.2.1: Oxide composition of the imaginary rock.
|
This is visualized in Fig.2.1, showing pie chart representations of the oxide composition of the example rock. The top row shows the proportions for weight percent, the bottom row for mol percent, in both cases with the overall composition to the left, and the contributions of the minerals in the rock to the right. Obviously the orthoclase feldspar contributes the bulk of the silica content, not the quartz. Although this is just an idealized example, it is not unrealistic, and similar proportions are found in many igneous rocks.
There is another interesting point to note: the "water content" of the rock. Because its molar weight is much lower than that of the other oxides, the contribution of the water to the overall weight is very low (0.9%), and this is a bit misleading, as its molar content is more than 3 times as high (3.3%). Of course, this rock does not contain any free water in the sense it contains free silica (as quartz). The water listed in the oxide composition of the rock is bound as hydroxyl groups (OH) in muscovite. However, upon changes in the environmental conditions, such bound water may play a role in chemical reactions or may be released from a mineral as free water. At high temperatures, water is a very aggressive and a very mobile agent that promotes many chemical reactions between minerals that would otherwise take place at a much lower speed. So even that little water may play a big role in a rock's chemistry. And - equally important, as we will see later - it has great influence on the physical properties of magma.
Silica, Silicates and Rock Chemistry
Now that the terms silica content and quartz content have been clarified, we can look at the relation between the silica content and the mineral composition of a rock. Again, we start with a simple example.If one melts a mixture of quartz SiO2 and magnesium oxide MgO in the right proportions in a pot, once the melt has cooled down and solidified again, one would yield a block of the silicate mineral forsterite, containing isolated SiO4 tetrahedra:

Fig.3.1: Oxide composition of forsterite
|
Because in the overall composition of the planet Earth, magnesium is a very important component and in many cases an indicator of the origin of a rock: forsterite is the principle component of olivine[3], a very common rock-forming mineral in the upper Earth's mantle. Certain mantle rocks, like peridotites and dunites, contain more than 90% olivine. If we look at the pie chart representation of the silica content of forsterite (Fig.3.1), we see that it is much lower (42.7 weight-% and 33.3 mol-% SiO2) than that of the granite shown above (Fig.2.1).
Of course it is much lower, you may say, that lies within its formula.
The interesting point is this:
Forsterite cannot form in the presence of quartz.
Quartz cannot form in the presence of forsterite.
Both minerals are mutually exclusive in a rock[4]. The reason for this behavior lies in an important property of silica: The SiO4 tetrahedra have a strong tendency to polymerize. They form larger groups by sharing oxygen atoms, either by addition of orthosilicic acid molecules, for example
[SiO4]4- + 2 H4SiO4 → [Si3O9]6- + 3 H2O + 2 H+ [3], a ring of 3 tetrahedra
or, under dry conditions, simply by the addition of silica, for example
So when silica reacts with metal oxides to form silicates, it can be incorporated into the new minerals as individual SiO44- groups, like in forsterite (formula [1]). But it might as well form chains, rings, sheets and networks of SiO4 tetrahedra. Whether it actually forms chains or networks, or just remains as individual SiO44- groups depends on many parameters, including the amount of silica present in the rock.
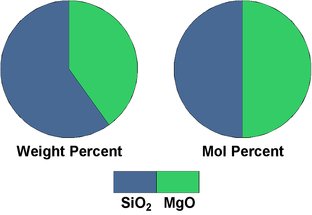
Fig.3.2: Oxide composition of enstatite
|
We get the mineral enstatite, a member of the rock-forming mineral group of pyroxenes. The higher silica content is shown as pie charts in Fig.3.2 (58.9 weight-% and 50 mol-% SiO2). In fact, enstatite and all the other pyroxenes are chain silicates, that is, their SiO4 tetrahedra form infinite parallel chains, just as suggested in formula [4] above.
The same result can be achieved by mixing forsterite with quartz:
So this is the reason why quartz and forsterite do not form together in a rock, they will react to form enstatite. And given what has been said about the abundance of olivines in the Earth's upper mantle, we may conclude that very likely there is no quartz in the upper mantle.
What if we add more quartz to the mixture? In this simple system of just 4 components, nothing: no other mineral than enstatite will form, and any extra amount of quartz will simply be left over in the mixture. Thus one can say: The presence of enstatite is compatible with the presence of quartz in a rock.
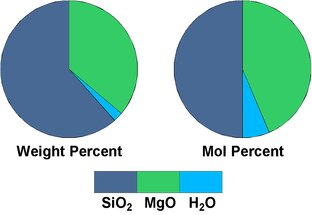
Fig.3.3: Oxide composition of anthophyllite
|
Anthophyllite is a member of the rock-forming mineral group of amphiboles, and like the pyroxenes, these are chain silicates, but while pyroxenes contain single chains of SiO4 tetrahedra, amphiboles contain double SiO4 chains and hydroxyl groups (OH). As shown in the pie charts in Fig.3.3, because water is also added, the silica content of the mineral is not increased as a whole, but relative to the MgO content.
We could do as we did in formula [6] and achieve the same result by mixing enstatite, water and silica:
If enstatite and quartz occur together as rock forming minerals in a rock, what does this mean? They probably cannot form together in the presence of free water, for any excess of silica would react with enstatite, and one would instead find a mixture of enstatite and anthophyllite. So the conditions during their formation have probably been rather dry. Of course, this is an idealized picture, and other minerals present in the rock could either promote or inhibit this reaction.

Fig.3.4: Oxide composition of talc
|
The structure of talc is very different from that of pyroxenes and amphiboles: it is a sheet silicate in which the SiO4 tetrahedra are arranged in two-dimensional infinite sheets and the metal and hydroxyl ions are placed between them. The differences in their structure are reflected in the differences in their physical properties: while forsterite, enstatite and anthophyllite are very hard minerals that may form centimeter-sized crystals, talc is very soft and made of tiny flakes, because the individual SiO4 tetrahedra sheets are almost freely movable relative to each other.
To summarize, because SiO4 tetrahedra tend to polymerize and arrange themselves in large groups, different minerals can form from the same metal oxides and silica, depending on the amount of silica in the rock, but also on other parameters, like the presence of water.
Basic Classification of Igneous Rocks
The term "igneous rocks" encompasses both volcanic (extrusive) and plutonic (intrusive) rocks. When classified by their overall chemical composition, most plutonic rocks have a volcanic counterpart of equal composition and vice versa. However, this is not necessarily true for the mineral content of these rocks. While, for example, in a granite the excess free silica is entirely contained in the quartz crystals, in a rhyolite (the volcanic counterpart of granite) all the free silica may be bound in a glassy matrix.Basic Terms Used for the Classification of Igneous Rocks
- The classification is based on mineral content
- The classification is based on based on rock chemistry
Which one is preferred is mostly a practical matter. Plutonic rocks are commonly classified by their mineral content, because it is possible to identify minerals and determine their relative content by optical methods, and very often even by visual inspection. Volcanic rocks, on the other hand, often consist of a fine-grained or even a glassy matrix with minerals embedded in them. This makes a visual identification of minerals impossible and one has to turn to chemical methods to determine the type of rock.
- acidic ↔ basic
- felsic ↔ mafic
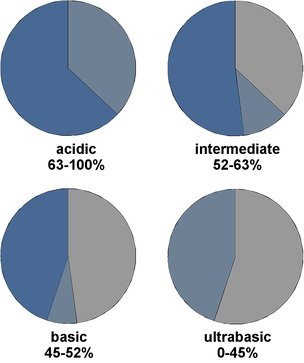
Fig.4.1: Classification of igneous rocks by their silica content
|
The term felsic, a word composed of the first syllables feldspars and silica, refers to generally light-colored minerals in an igneous rock that are solely composed of Si, Al, K, Na, Ca, and O. This includes feldspars and quartz, of course, but also the so-called foids, short for feldspathoids. Foids partially or completely replace feldspars in rocks of low silica content. They are similar in appearance to feldspars, but they differ in their structure and have a lower SiO2 content. The presence of foids in a rock is a safe indication that there is no quartz in this rock.
The term mafic originally referred to minerals rich in magnesium and iron (ferrum in Latin). These minerals are often dark, in particular if they contain a lot of iron. But mafic minerals are more openly defined and include any minerals that are not felsic, like micas, pyroxenes, amphiboles, garnets, oxides and minor components of a rock, like zircon, apatite, etc. Rocks that contain more than 90% mafic minerals are called ultramafic rocks.
Bright-colored rocks dominated by felsic minerals tend to be acidic rocks, and rocks with a low content in felsic minerals (and thus high content of mafic minerals) tend to be basic rocks. But in particular for felsic minerals this relationship is not always true.
The QAPF Classification
A generally accepted classification scheme that is based on the mineral content is the QAPF scheme, introduced by Streckeisen in 1974. The classification is based on 5 groups of minerals:
Quartz
Alkali-Feldspar
Plagioclase Feldspar
Foids
Mafic minerals
To classify an igneous rock, only the felsic mineral components are considered and the relative content of Quartz, Alkali-Feldspar, Plagioclase, and Foids within the felsic content is determined, the mafic components are ignored. This scheme is only applied to rocks with at least 10% of felsic minerals (= less than 90% mafic minerals).
For practical reasons, the values are given as volume percent: the rock is analyzed in a petrographic microscope, in which the relative volumes of the various minerals are optically determined. Since the specific density of the felsic minerals is mostly between 2.5 and 3.0, the numbers do not differ too much from weight percentages.
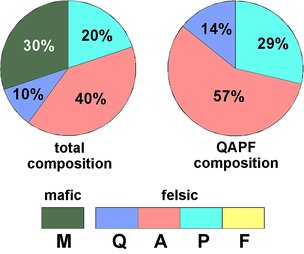
Fig.5.1: Example for the relation between the total composition and the QAPF composition of a rock
|
So if a rock is made of 30% mafic and 70% felsic minerals, and contains 10% quartz, 40% alkali-feldspar and 20% plagioclase, the QAPF values given for quartz, alkali-feldspar and plagioclase are 14%, 57%, and 39%, as shown in Fig.5.1. This would be a granitoid rock, a so-called "quartz-bearing syenite", and the mafic minerals might be various micas, amphiboles, garnets, etc.
Because foids and quartz are mutually exclusive in a rock, the QAPF classification is always based on a maximum of 3 components.
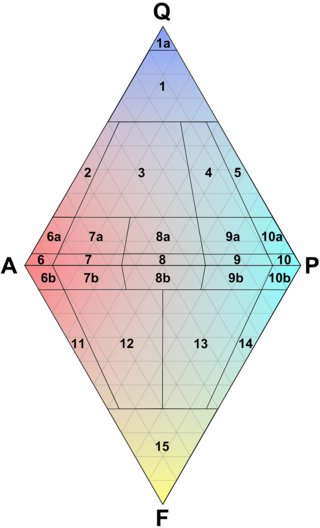
Fig.5.2: The QAPF Diagram for Igneous Rocks
|
The QAPF diagram is divided into 15 fields that define ranges of mineral compositions for the different classes of rocks. Some of the fields are subdivided (see Table 5.1 below for a partial assignment of rock names to the numbers, and a full list at the appendix The QAPF Diagram).
Every rock whose QAPF composition falls in the range of the upper triangle contains some quartz, every rock type defined in the lower triangle is void of it and contains foids instead. Rocks with compositions along the A-P line contain only feldspars and mafic minerals.
Probably the most important field for the quartz collector is field 3, the field for granites. A granite is defined as follows:
"Any intrusive igneous rock with less than 90% mafic minerals, a content of 20% - 60% quartz and between 90 % and 35% of alkali-feldspar in the remaining volume."
This, of course, is a lengthy definition, and one benefit of using the QAPF diagram is that it is much easier to memorize the locations of the fields in the QAPF diagram than to memorize all the lengthy definitions of 15 different rock types.
The other benefit of the QAPF diagram is that one recognizes systematic neighborhood relationships between the rock types, for example, how a monzonite differs from a syenite or a granite.
Table 5.1 below lists a few important plutonic rocks and their volcanic counterparts. A full list of all rocks defined in the fields in the QAPF diagram is found in the appendix The QAPF Diagram. Rocks that are most common and occur in large volumes at the surface are marked bold. Interestingly the silica rich plutonic rocks granite and granodiorite are more common than their volcanic counterparts rhyolite and dacite, whereas the silica-poor volcanic rocks andesite and basalt are much more common than the plutonic rocks gabbro, diorite and anorthosite.
Some of the fields are assigned to two or three rock types (for example field 10), these rocks are distinguished by their mafic mineral content.
| Field Number | Plutonic Rocks | Volcanic Rocks |
|
1a 3 4 5 7 8 9 10 11 14 |
Quartzolite Granite Granodiorite Tonalite Syenite Monzonite Monzodiorite / Monzogabbro Diorite / Gabbro / Anorthosite Foidsyenite Foiddiorite / Foidgabbro |
- Rhyolite Dacite Plagidacite Trachyte Latite Andesite Basalt Phonolite Tephrite / Basanite |
Igneous Rocks
Quartz occurs as a rock-forming mineral in igneous rocks if the overall silica content of the original magma surpasses 63-65 weight-percent. When the magma cools, different minerals will successively grow in the still liquid environment[5]. While certain ions get concentrated in the mineral grains, the silica and the water content of the residual liquid increases. One of the last minerals to form is quartz, either as its high-temperature polymorph, beta-quartz, or as regular alpha-quartz. Because it simply fills out irregular spaces between already present feldspar and mica crystals, it usually does not develop crystal faces[6]. The longer the cooling of the magma takes, the larger the final mineral grains will be. Large bodies of intrusive igneous rocks that do not appear at the earth's surface cool very slowly and turn into granites and related igneous rocks with their well-known "pepper-and-salt-structure". Extrusive igneous rocks (better known as volcanic rocks) with a similar chemical overall compositions are rhyolites. If the magma cools very quickly, there is not enough time for a differentiation of the magma into different minerals and the formation of crystals, and a natural glass, obsidian, will form that does not contain any quartz.
Intrusive or Plutonic Rocks
Intrusive igneous rocks are easily recognized by their texture: they are composed of irregularly intergrown mineral grains of different sizes and colors. The majority of igneous rocks are of granitoid composition and contain quartz, granites, granodiorites, quartz monzonites and quartz monzodiorites are most common.

20mm 1488x992 453kb |

20mm 1040x760 163kb - 2080x1520 537kb |

|

|
Intruded plutons may merge into a very large body that extends over hundreds of kilometers, called a batholith. Batholithes are typically found where an oceanic plate subducts under a continental plate. Complex processes of magmatic differentiation at these plate boundaries lead to an enrichment in silica and water, a lowering of the melting point and a decrease in specific density of the magma. As a consequence, the magma starts to rise along the plate boundary and either intrudes overlying rocks as granite-type rocks or erupts at the surface to form volcanoes.

|

|

|
In dry climates weathered granite often assumes bizarre, rounded shapes that develop because the surface peels off in thin onion-like sheets. The image shows a granite formation near the Gross Spitzkoppe, Namibia. As a scale note the tree to the left.

|

|

20mm 2320x1472 407kb |
Volcanic and Subvolcanic Rocks

|
Figure 7.8 shows Damavand, 5610 m, a typical composite volcano in the Alborz mountains north-east of Tehran, Iran. It is made of of intermediate volcanic rocks, mostly trachytes and andesites.

20mm 2080x1520 536kb - 4160x3040 1482kb |

20mm 1776x1504 423kb |

10mm 2288x1600 506kb - 4576x3200 1532kb |

20mm 1920x1568 517kb - 3840x3136 1411kb |
This rock is as acidic (silica-rich) as its granitoid counterparts, so there is a good chance to find quartz varieties in cavities. If these actually form or not also depends on the water content of the magma, or the availibility of hydrothermal brines from other sources, as water promotes the alteration of the rock, the formation of cavities during cooling and the dissolution and transport of silica through the pores of the rock. This rock contained only very few very small cavities with small quartz crystals, it was apparently very dry during its solidification. From a subvolcanic dome in the Karkas Mountains at Josheghan Qali village, Isfahan Province, Iran.

|

20mm 2320x1552 534kb |
- it forms macromolecular chains and networks of SiO4-groups and thereby increases the viscosity
- it inhibits the diffusion of ions and thereby inhibits the growth of crystals
As a result, silica-rich lava is tenacious and flows slowly, and also has strong tendency to solidify as a volcanic glass. The glass may be massive, as the well-known obsidian, but the spongy pumice is also a volcanic glass. In line with their high silica content, many obsidians are rhyolitic in composition, and most of them contain a small amount of water, too (as molecular water as well as bound in silanole groups). By contrast, basaltic lava will form volcanic glass of comparable homogeneity only if it is cooled very quickly. For example, the rims of pillow lavas are made of volcanic glass that formed when the lava was quenched by sea water. However, the matrix of intermediate and basic volcanic rocks with a porphyric texture does often contain a large amount of glass or even consist entirely of glass.

20mm 2320x1552 448kb |
Volcanic glass is generally not a stable rock. At near-surface conditions, forms with a large surface (pumice, for example) tend to weather, while the massive obsidian is more stable. When a volcanic glass stays hot or is heated up by successive lava flows, it may experience devitrification ("un-glassing"). Then minerals (that is, crystals) grow inside the glass matrix, forming gray or white patches and rims. Unaltered obsidian and pumice deposits are generally young, rarely are they older than a few million years.

20mm 2288x1360 628kb |

10mm 1744x1504 221kb - 3488x3008 590kb |

20mm 1584x1520 384kb - 3168x3040 1055kb |

5mm 1680x1424 416kb |

20mm 1000x788 143kb - 2000x1576 480kb |

5mm 1080x717 183kb - 2160x1434 562kb |
Sedimentary Rocks
Quartz tends to accumulate in deposits of eroded material, both due to its physical and chemical resistance and because it is often formed from silicate minerals during chemical weathering. If you ever traveled through a desert, you might have noted that often the mountains are much darker than the debris surrounding them. The darker components of igneous and metamorphic rocks consist mostly of minerals that easily weather under the influence of carbonic acid, oxygen, and water. Micas and feldspars will slowly decompose and their tiny flakes and grains will be carried away by the wind, leaving quartz behind. Once quartz has formed and appeared at the surface, it will only slowly weather away, if at all. Most of the weathering of quartz is due to physical forces: changes in temperature, erosion, cracking by ice-wedges, and grinding. Not surprisingly, many sedimentary rocks, in particular those that formed on land, contain large amounts of quartz.Chemical weathering and dissolution of quartz substance only plays a role in tropical semi-arid and savanna climates. Here quartz slowly gets dissolved and washed out of top soil and regolith, and the residual iron and aluminum oxides and hydroxides often form economically important bauxite deposits.

10mm 1200x1136 203kb - 2400x2272 582kb |
Sandstone is often a very porous material with a rough fracture surface. Depending on the cement and the shape of the grains, it can be quite tough or almost crumbly.
The photo shows a piece of bright-colored Triassic quartz sandstone from Unteralpfen in the Southern Black Forest, Germany. It is mostly made of millimeter-sized quartz grains and the voids in between are partially filled with yellow iron oxides and calcarine material.

20mm 1159x760 159kb - 2318x1520 595kb |

|

|
That the rock is porous can be concluded from the fact that a little tree grows on top of it. It wouldn't if the rock did not store any water from rainfalls. Sandstones are important aquifers.
The soils formed on quartz sandstones are usually rather infertile. They do not contain important trace elements needed as nutrients, and even if they did, they do not contain micas or produce[7] clay minerals that usually serve as a storage and buffer for these elements.

|

|
Chert, a microcrystalline quartz variety, can also be a rock-forming material. It may form by alteration of volcanic rocks, for example, from silica-rich ashes and debris in the Onvervacht sedimentary group in the Transvaal province, South Africa ( ->Lowe and Knauth, 1977).
Most cherts are based on biogenic sediments, however. Novaculites, diatomites and radiolarites are rocks of biogenic origin with a very high silica content that form from marine and lake sediments containing opaline skeletons - the same that are also the main silica source of flint nodules in sedimentary rocks. The opaline substance in the rocks is slowly altered under the influence of percolating waters and slowly recrystallizes into other silica polymorphs, like cristobalite, moganite, and ultimately quartz. Thus older cherts typically have been completely transformed into quartz (still cryptocrystalline) and do not contain any opaline substance. This transformation is accompanied by a change in texture: the initially irregular grain borders get straightened out and form a "triple-point texture" (see Knauth, 1994, for a review on chert formation).

|
The opaline skeletons of marine single-cell organisms were deposited on the ocean floor and later solidified into a fine-grained chert-like rock, sometimes entirely made of chert. Although the skeletons are up to about a millimeter in size, the former skeletons are usually not visible to the naked eye. In radiolarites from Mesozoic and Paleozoic ages the opaline substance has been completely converted to quartz.
Figure 8.5 shows an outcrop of radiolarite in an old radiolarite quarry (now a historic site) at Lautenthal in the Harz Mountains, Germany (the field of view is about 240x360 cm). The radiolarite was formed in a deep marine environment at the Lower Carboniferous age about 350 million years ago. During the Harz orogeny it was subject to intense folding. Interspersed within the radiolarite are a few sheets of slate which can be recognized by their hatched texture and the fact that they have eroded more than the radiolarite.

20mm 1152x784 134kb - 2304x1568 491kb |

1mm 1076x712 160kb - 2152x1424 652kb |

1mm 1000x704 136kb - 2000x1408 504kb |

10mm 1104x816 144kb - 2208x1632 544kb |

20mm 2304x1552 464kb - 4608x3104 1316kb |
Diatomites are rocks similar to radiolarites that also form from sediments of opaline skeletons, but the skeletons are mostly those of diatoms, single-celled algae. Diatoms occur in marine and in sweet-water environments, so diatomites can also found in former lake beds.
The deposition of large amounts of biogenic opaline silica in deep water marine environments and the formation of diatomites is a relatively new phenomenon in the history of Earth - only since about 50 million years the diatoms flourish, likely because of a general cooling of the climate (Knauth, 1994). In present-day oceans, siliceous ooze can be found in areas of high biological activity, but only at great depths under conditions that - due to high pressure and low temperatures - do not allow the deposition of calcite and calcareous skeletons and shells.
If the diatomite is very young and has not been buried under other rocks at great depths for a long time, it has not yet been compressed and sometimes remains very porous. This type of diatomite is also called diatomaceous earth because of its soft consistence.

|
Diatomite varies in composition, it may be almost pure amorphous (opaline) silica, but slowly - on a geological time scale - transforms into chert composed of quartz. Some diatomites contain cristobalite, and prolonged exposure to the fine dust of cristobalite may cause silicosis and lung cancer (e.g., Goldsmith, 1994, Pelucchi et al., 2006).

20mm 1728x1424 486kb |

20mm 1912x1504 400kb |

20mm 2160x1424 307kb - 4320x2848 955kb |
Metamorphic Rocks
The role of quartz in metamorphic rocks is very complex, because the silica content can determine the faith of a rock during metamorphosis to a large extent. During low-grade metamorphosis, at low temperatures and pressures, the quartz content of the resulting rock will not differ greatly from that of the original rock, as quartz is still an almost inert substance under these conditions, but at higher temperatures quartz will start to react with other minerals to form silicates. So while it may coexist with some minerals at certain conditions, it may simply disappear from the rock under different conditions, for example upon prolonged heating. Other factors that determine the reactivity and the fate of quartz in a rock are the water content, the amount of carbon dioxide and oxygen, etc.Rocks that consist mostly of silica and carbonates shall serve as an example, because the processes in them are simple, at least when compared to processes in silicate rocks. Limestone, a sedimentary rock, is largely made of calcite, CaCO3. Quartz and calcite can coexist over a wide range of conditions, and quartz crystals found in limestones are fairly common. For example, certain types of authigenic quartz form inside limestone. Metamorphosis at moderately high temperatures will turn a limestone into marble, and quartz crystals can also be found in that type of rock, like the famous Carrara diamonds.
However, at temperatures above about 450°C, quartz may start to react with calcite to form the mineral wollastonite. Ca3Si3O9:
This reaction releases carbon dioxide, and depending on the amount of CO2 present (the partial CO2 pressure, to be exact), the reaction can be inhibited until higher temperatures are reached. As a consequence, that reaction is self-inhibiting. There is also a dependence from the total pressure, as well as the partial H2O pressure, and quartz and calcite can remain stable at temperatures of more than 700°C in the absence of water. The reverse reaction cannot take place if the CO2 has largely escaped from the rock and the quartz will not be formed again when the rock cools. The fact that CO2 is removed from the system and "freezes" the reaction products makes silicate-carbonate reactions and the minerals formed in them important indicators of conditions during metamorphosis.
Of course, whether any quartz remains after high-grade metamorphosis also depends on the initial amount of quartz in the rock. Quartz might have been present in excess, so not all of it has been consumed. One should also keep in mind that these reactions are extremely slow, and many rocks are not "mature", that is, the chemical reactions have not been completed and the mineral components have not reached a chemical equilibrium.
If a rock contains large amounts of dolomite, MgCa(CO3)2, things get more complex. In general, quartz is far less stable in dolomite marbles than in calcite marbles and will start to react with dolomite at about 400°C. A common product of quartz, dolomite and water is talcum, Mg3[(OH)2/SiO4], a sheet silicate:
At higher temperatures, the talcum will react with calcite and quartz to form the mineral tremolite, Ca2Mg5[(OH)2/Si8O22], an amphibole, again releasing CO2:
In a further step tremolite may consume more quartz and calcite to form the mineral diopside, CaMgSi2O6, a pyroxene:
It is important to note that the minerals on both sides of formulas [10] to [13] may coexist in a rock, and that there are overlapping areas of stability for most of the minerals involved in the reactions. So one may -depending on the local conditions- find quartz along with diopside and tremolite, or talcum together with quartz and tremolite.
The whole matter gets even more complex in rocks that contain alumosilicates in addition to carbonates and silica, like marls and their derivative metamorphic rocks.

20mm 1600x1007 278kb - 3200x2014 807kb |
Quartzite is a metamorphic rock[8] that is made of at least 80% quartz. Quartzites are formed from precursor rocks that are mostly made of quartz, this includes sandstones, cherts and radiolarites. In most cases it is simply a sandstone that has "matured" into a very tough rock composed of tightly interlocked quartz grains and that has undergone a low-grade metamorphosis at low temperatures. In most sandstones individual grains can be identified with the unaided eye, whereas quartzite looks much more homogeneous and its fracture surface does not show a dull, rough grainy structure. Instead, the fracture cuts right through the individual quartz grains and can be a little shiny. While in sandstone individual grains are mostly rounded, the grains in quartzite are more angular and as the former voids in sandstone have been filled with quartz substance during the recrystallization of quartz grains, quartzite is not porous. It is also much less susceptible to weathering in moderate and cold climates and forms steep cliffs and outcrops in a landscape. Quartzite is generally more translucent than sandstone and looks a bit like marble. Being much harder and also very tough, it is easy to distinguish from marble. Quartzite often contains small amounts of muscovite mica.

50mm 1160x760 134kb - 2320x1520 490kb |

20mm 1120x760 136kb - 2240x1520 465kb |

5mm 1080x717 183kb - 2160x1434 553kb |
A gneiss is a metamorphic rock that shows signs of layering and alignment of structures parallel to the layering. The classification of a rock as a gneiss is primaly based on its texture and not its composition, but a gneiss should contain a significant amount of feldspars. There is no strict definition for the minimum amount of feldspar required to make it a gneiss (Vinx, 2003). The term "gneiss" subsumes a great variety of rocks and there are gradual transitions to schists as well as intrusive igneous rocks (granitoid rocks). A common denominator of all gneisses is their tendency to part into plates that are thicker than 1 cm (Vinx, 2003), whereas shists and phyllites tend to form thin sheets. Depending on the precursor rock, orthogneiss that is derived from ingeous rocks and paragneiss that is derived from sedimentary rocks are sometimes distinguished. Not all gneisses contain quartz, but most do, since the precursor rocks are often granitoid rocks or quartz-bearing sedimentary rocks.

20mm 1632x1856 525kb |

20mm 1080x760 177kb - 2160x1520 564kb |
Slates, phyllites and mica shists are layered metamorphic rocks that are derived from sedimentary rocks with clay minerals, often from deep water marine environments. Their presence always indicates mountain forming processes (orogenies). Slates are composed of sheet-silicates and quartz and have undergone only a weak, low-grade metamorphosis. In phyllites, which form at low-grade metamorphosis (greenshist facies), the mica crystals are so fine that they can hardly be seen with the unaided eye. They are composed mainly of micas (50% or more), quartz and carbonates. Mica shists form from phyllites during medium grade metamorphosis (amphibolite facies) and look more coarse grained. Their mineral composition is similar, but they also often contain conspicious accessory minerals like garnets and amphiboles.
Many mica shists and phyllites contain large amounts of fine grained quartz. If these rocks or their precursor rocks are subject to tectonic stress, quartz tends to segragate from the rock and accumulates in fine-grained, white lense-shaped bodies or irregular elongated veins that run roughly parallel to the layers of the rock. The quartz is then sometimes called segregation quartz, and its presence is an indication of a long term, weak to medium grade metamorphosis. A few in situ images of segregation quartz are shown in the chapter Occurrence.
During higher grade metamorphosis mica shists will gradually transformed into paragneiss, and now the mineral composition changes at the expense of quartz - the quartz reacts with the mica minerals to form other minerals, for example feldspars and andalusite (or sillimanite, depending on the pressure):
muscovite + quartz → K-feldspar + andalusite + water
Which is one of the reasons why many paragneisses have a lower overall quartz content.

50mm 1080x704 157kb - 2160x1408 566kb |

20mm 1160x708 153kb - 2320x1416 526kb |

50mm 2176x1596 601kb |

10mm 1496x1536 310kb |

10mm 2176x1552 459kb - 4352x3104 1450kb |
Quartz-Free Environments
Although quartz is one of the most abundant minerals and makes up an estimated 12% of the Earth's continental crust, it is very unevenly distributed, and most of the quartz is concentrated in granites, gneisses, quartzites and sandstones. Certain types of rocks are completely void of quartz and free silica.There are different ways to interpret this lack of free silica:
- The lack of quartz in an igneous rock might be solely attributed to the bulk chemical composition of the magma it formed from: if the concentration of SiO2 is too low, it does not allow for quartz to form as a mineral, simply because there is no silica left over from the mineral forming processes.
- It can be viewed as being caused by the presence of other minerals in the rock that react with the quartz. This, of course, also depends on conditions other than the bulk chemical composition: quartz might be stable inside a rock at a certain temperature or pressure, but upon changes the silica might be used up in reactions with other minerals that lead to the formation of new silicate minerals. That way the overall chemical composition of the rock does not change, but its mineral composition does and quartz may appear or disappear in it.
These interpretations are not mutually exclusive, they are just different ways to express the same fact.
Sedimentary Rocks
As quartz is almost inert at temperatures and pressures that produce sedimentary rocks, the most important factor that determines if quartz is present in them is the presence of free silica during the rock's formation.
Normally, quartz will not disappear from these rocks. If one chooses a very open definition of the term "sedimentary rock", then there is one notable exception: In tropical climates with pronounced rain and dry seasons, silica (free silica as well as silica bound in silicates) is slowly washed out of the soil and the remaining minerals (aluminum and iron oxides) form a very infertile soil, laterite, that might later assume a hard, brick-like consistence.
However, weathering of minerals contained in sedimentary rocks may release silica.
Igneous Rocks
The amount of silica (including the silica that is chemically bound in silicates) in igneous rocks is one of the parameters that is used for their classification. Above the terms felsic and mafic have already been introduced. Free silica (as quartz) can be expected to be found in rocks with more than about 60-65% silica in their total chemical composition. Well known examples are granite and its volcanic rock counterpart, rhyolite.
Another important classification scheme has also been presented above: the QAPF diagram, which uses the quartz content instead of the silica content of rocks as a parameter for classification. The rocks represented by the lower part of the QAPF diagram are free of quartz and contain characteristic minerals that replace feldspars in these rocks, so-called "feldspathoids" or foids. The presence of foids in a rock is a safe indication that this rock is free of quartz. Note that foids are still called felsic minerals. A well known example of a foid is the blue sodalite, Na4Si3Al3O12Cl, with a silica content of only 37 weight-%.

Fig.10.1: Chemical composition of typical feldspars and foids compared.
|
Fig.10.1 shows pie charts of their chemical composition (in weight percent) in comparison with two common feldspars, orthoclase and albite, NaAlSi3O8. The foids contain less silica, and if they get in contact with quartz, they will be slowly transformed into feldpars:
Leucite + Quartz → Orthoclase
Foid + Quartz → Feldspar
Quartz crystals might still grow in pockets and veins from silica dissolved in hot waters that percolate a weathered foid-bearing rock, or small amounts of quartz might fill out the voids between the grains of an altered rock (then invisible). So the rule that foids and quartz are incompatible is only true for mature and fresh rocks in chemical equilibrium. If, by some odd coincidence, foids are found next to quartz, the foids will be separated from the quartz by a thin layer of feldspars. However, it is most unlikely to find well formed crystals next to foids.
In addition to these foids, silica-poor rocks often contain large amounts of dark minerals rich in magnesium and iron (ferrum in Latin), mafic minerals. Many of these minerals are either incompatible with the presence of quartz or are loosely associated with silica-poor environments, like pyroxenes and amphiboles (see Silica, Silicates and Rock Chemistry above).
Among the dark minerals in general, micas (like the members of the biotite group) and iron compounds (pyrite, hematite, limonite, magnetite) are fully compatible with the presence of quartz, and may occur in very acidic rocks.

20mm 1568x1088 249kb - 3136x2176 749kb |
The peridotite and the basalt (probably a basanite or tephrite that also contains small grains of olivine) both have a very low content of SiO2 compared to that of other igneous rocks like granite, diorite or andesite. A fresh and unaltered peridotite or a basalt containing an olivine fraction will not contain any free silica, and of course, no quartz. If it does, the quartz has certainly entered the basalt later at relatively low temperatures and did not remain in the rock for a long time. If it had been swallowed by a basaltic magma, it would have simply been dissolved in the magma to form new minerals.

20mm 1520x1007 319kb - 3039x2014 924kb |
Metamorphic Rocks
Because the relative amount of SiO2 in a metamorphic rock has a profound influence on the types of minerals that form in them, the presence or absence of certain minerals can indicate if quartz can be found in them. The following minerals do not form at the presence of quartz or sufficient amounts of SiO2 in the originating rock:
- silicates with a low portion of SiO2 and a relatively high concentration of magnesium, Mg, like:
Forsterite, Mg2SiO4, an olivine mineral
- oxides and carbonates of magnesium, like:
Periclase, MgO
Magnesite, MgCO3, at medium- and high-grade metamorphosis
Dolomite, CaMg(CO3)2, at high-grade metamorphosis
- silicates with a low portion of SiO2 and a relatively high concentration of aluminum, Al, like:
Corundum, Al2O3, at medium- and high-grade metamorphosis
Foids in general, like leucite, K[AlSi2O6], analcime, Na[AlSi2O6]⋅H2O, nepheline KNa3[AlSi2O6]4
A mineral that doesn't get along too well with quartz is corundum, Al2O3, better known under the names of its varieties ruby and sapphire. If the rock is in chemical equilibrium, that is, if there has been sufficient time for its components to fully react with each other, corundum and quartz will react to form either sillimanite, andalusite, or kyanite:
In low-pressure environments quartz can coexist with all the Al2SiO5 polymorphs, but not with corundum. Corundum and quartz can be found together in sedimentary rocks, which is possible because both Al2O3 and SiO2 are not very reactive substances and the reaction takes place at a very low speed.
Although a stability field in the area above 650°C and 3.5 kbars has in been predicted by experimental studies on the system Al2O3, SiO2 and H2O (Aramaki and Roy, 1963), these findings could not be reproduced in a later study (Carr, 1968) and were attributed to the use of amorphous chemicals (silica, aluminum oxide) as starting substances, and until recently there was a general agreement that corundum and quartz never form together and never are in chemical equilibrium.
But in fact there are a few high-grade metamorphic environments in which quartz and corundum, or quartz and spinel (which is almost as "quartz-unfriendly"), are found together (for a review of localities, see Mouri et al., 2004). In most cases, the quartz and corundum grains are separated by a narrow rim of sillimanite or kyanite, but in some cases corundum and quartz are in immediate contact and apparently in chemical equilibrium. These environments are all characterized by very high pressures (between 7 and 17 kbars) and temperatures (between 900°C and 1100°C), so there seems to be a well-defined stability field of the coexistence of quartz and corundum. In fact, the presence of quartz and corundum grains in immediate contact in a metamorphic rock is seen as an indicator of extreme high-pressure and temperature conditions.

20mm 1136x1072 222kb - 2272x2144 609kb |
Further Information, Literature, Links
C.M.R. FowlerThe Solid Earth - An Introduction to Global Geophysics
Cambridge University Press, Cambridge, 2004
ISBN 0-521-89307-0
Gregor Markl
Minerale und Gesteine - Eigenschaften - Bildung - Entstehung
Elsevier, Spektrum Akademischer Verlag, Heidelberg, 2004
ISBN 3-8274-1495-4
Martin Okrusch, Siegfried Matthes
Mineralogie - Eine Einführung in die spezielle Mineralogie, Petrologie und Lagerstättenkunde.
Springer-Verlag, Berlin Heidelberg New York Tokyo, 2005
ISBN 3-540-23812-3
Roland Vinx
Gesteinsbestimmung im Gelände
Elsevier, Spektrum Akademischer Verlag, Heidelberg, 2003
ISBN 3-8274-1513-6
Hans-Rudolph Wenk, Andrei Bulakh
Minerals - Their Constitution and Origin
Cambridge University Press, Cambridge, 2003
ISBN 0-521-52958-1
Footnotes
1 The oceans cover 70.6% of the Earth, so one would expect that value to be 29.4%. But the continents are larger than they appear, because parts of the continental crust are hidden in the marine continental shelf areas that are covered by water.2 Material in alluvial rocks and sediments has been transported and deposited by rivers and streams.
3 Olivine is the name for a group of minerals that encompasses forsterite, Mg2SiO4, and fayalite, Fe2SiO4. Olivine is typically made of 85%-95% forsterite and 5-15% fayalite. The gemstone peridote is just a deeply green olivine.
4 Occasionally forsterite or olivine is found next to quartz in a rock, but they did not form together. This happens, for example, when olivine-rich and quartz-rich sediments get mixed and solidify, or when a olivine-rich lava encloses quartz-rich rocks.
5 In most cases, the magma is not a liquid in the common sense, but a mixture of highly viscous melt and small crystals of various minerals.
6 In rare cases one can see the hexagonal outlines of quartz crystals that formed as beta quartz in granites and rhyolites.
7 Clay minerals are produced during the weathering of feldspars.
8 Sometimes the term "quartzite" is also used for some sandstones.
9 The shists and phyllites of that locality are more commonly referred to as mylonites and ultramylonites to indicate that they formed by mechanical grinding and recrystallization of the precursor rocks.
10 The label that came with the rock said it was made of kryptoperthitic K-feldspar, biotite, quartz, and corundum. That did not make sense to me, as this was a high-grade metamorphic rock with a "mature" look and the corundum did not show any signs of alteration. Sebastian Möller at the university of Kiel kindly analyzed a thin section of the specimen using a petrographic microscope and found the rock to be made of perthitic and non-perthitic feldspar, biotite, corundum and small amounts of zircon, but no quartz.
 Printer Friendly Version
Printer Friendly VersionCopyright © 2005-2014, A.C. A k h a v a n
Impressum - Source: http://www.quartzpage.de/gen_rock.html

